Engineering & Validation Services
| Period: | Projects: | Location: |
 |
| 2004 - until today | Projects for large pharmaceutical companies | Germany, Switzerland | |
| Scope: |
|
||
| To the processes: |
|
||
|
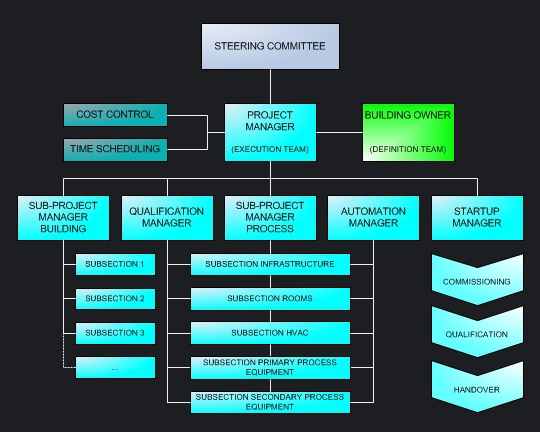
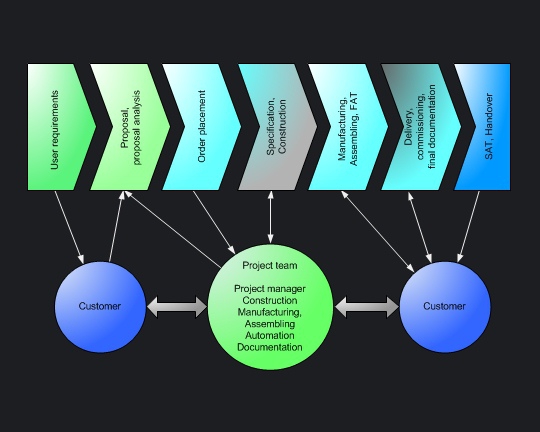
Global engineering guidelines represents the basis to manage project in large pharmaceutical companies. Location-specific standard operation procedures shall provide a framework covering the technical project processing. Within the scope of these projects the companies outsorce individual tasks by awarding a contract. |
|||
|
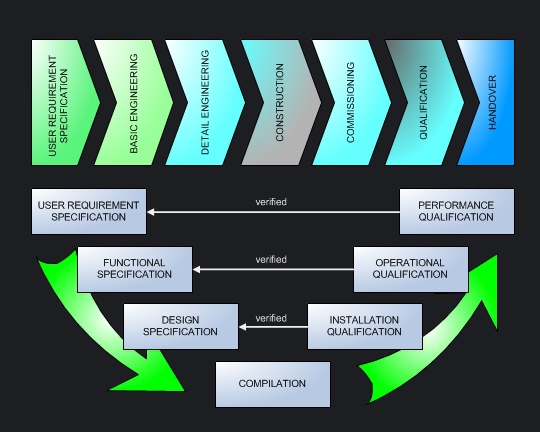
Qualification is conducted parallel to all phases of the project, in accordance with the v-model as used in software development. European-influenced companies often leads extensive design qualification and to that effect they need supplier specifications at an early stage of the projects. |
 |
| Period: | Projects: | Location: |
 |
| 1990 - 2003 | Projects for medium sized pharmaceutical companies | Germany | |
| Scope: |
|
||
| To the processes: |
|
||
|
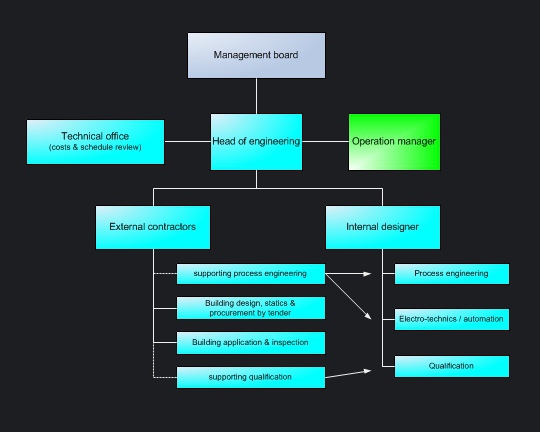
Project in group-free small to mediumsized companies were processed by the technical manager as part of his responsibilities. The project team is mainly staffed by own employees. Sectoral planning services will be externally-hired. |
|||
|
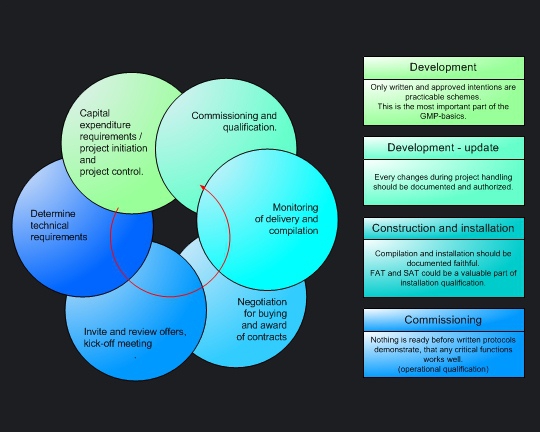
Streamlined corporate structures, smaller project organizations and the lack of company-wide processes enables shorter decisions. However, projects organised by mediumsized companies meets all necessary GMP-Requirements. Project procedures are oriented toward five simple guidelines:
|
 |
|
Whoever delivers capital goods to the pharmaceutical industry should be well-versed in GMP-Requirements - within medium-sized firms as within large pharmaceutical companies. Whatever your pharmaceutical customer needs, the project phases
are significantly influenced by GMP-Regulations. The more process understanding and GMP experience a supplier acquires, the better he protect themselves competitive advantages. |
 |
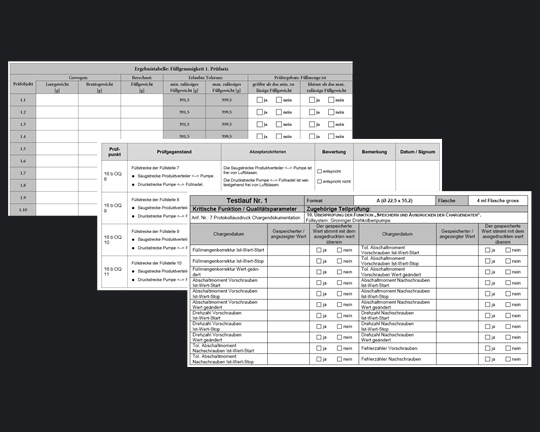
QualificationWhatever you deliver to a pharmaceutical customer, your corresponding documentation e.g.
are significantly influenced by essential GMP-Compliance. The more GMP-Profile a supplier acquires, the better he protect themselves competitive advantages. |
 |
